Journal of Animal Health and Production
Case Report
Surgical Approach for Vaginal Hyperplasia and Vaginal Fold Prolapse in Bitch
Ankit Kumar Ahuja*, Shahbaz Singh Dhindsa, Ajeet Kumar, Prahlad Singh
Department of Veterinary Gynaecology and Obstetrics, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab. India.
Abstract | Majority of growths in the vaginal canal of bitch are contributed by vaginal prolapse, vaginal hyperplasia and vaginal tumors. Hormonal profiling of canine during whelping is suggestive of reduced serum progesterone concentration and increased estrogen concentration. Present case reports modified technique for surgical excision of high grade vaginal prolapse and vaginal hyperplasia in the bitch. In this case, surgical excision performed was successful and ovariohysterectomy was avoided to maintain the reproductive ability of the animal. Follow up of the case represented healthy recovery without any reoccurrence during subsequent estrus.
Keywords | Vaginal hyperplasia, Vaginal fold prolapse, Circumferential incision diestrus, Hormonal
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | July 17, 2018; Accepted | August 20, 2018; Published | September 26, 2018
*Correspondence | Ankit Kumar Ahuja, Department of Veterinary Gynaecology and Obstetrics, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab. India; Email: ankit.ahuja1947@gmail.com
Citation | Ahuja AK, Dhindsa SS, Kumar A, Singh P (2018). Surgical approach for vaginal hyperplasia and vaginal fold prolapse in bitch. J. Anim. Health Prod. 6(3): 86-89.
DOI | http://dx.doi.org/10.17582/journal.jahp/2018/6.3.86.89
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2018 Ahuja et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Most common growths of vaginal canal of bitch are vaginal prolapse, vaginal hyperplasia and vaginal tumors (Manothaiudom and Johnston, 1991). Protrusion of edematous vaginal floor or vaginal folds from the vulvar opening is called vaginal prolapse. It usually occurs during estrous and near whelping. Prolapse of vagina can also occur due to vaginal tumors (Williams et al., 2005) or from any injury to the genitalia (Sananmuang et al., 2017). During estrous, edematous swelling and protrusion of the vaginal mucosa occur due to high levels of estrogen (Johnston et al., 2001). The condition is generally characterized as vaginal hyperplasia and vaginal prolapse.
Hormonal profiling of canine during whelping are suggestive of reduced serum progesterone concentration and increased estrogen concentration (Johnston et al., 2001; Konig et al., 2004; Rani et al., 2004). Hence the chances of vaginal prolapse are comparatively higher in pregnancy and low in dioestrus (Johnston et al., 2001; Okkens, 2001). Complete exteriorization of cervix occurs in complete vaginal prolapse but not with partial prolapse (Wykes, 1986). There is a breed predisposition especially brachycephalic breed, Drahthaar, Kurzhaar, Caucasian Shepherd, Doberman (Feldman et al., 2004, Mostachioet al., 2007) appears to be more prone to vaginal fold prolapse and that may be due to hereditary weakness of the perivulvar tissue. Other etiological factors include constipation, forced separation during coitus and incompatibility in size during breeding can also contribute to the development of vaginal prolapse (Purswell, 2000).
Vaginal prolapse is categorized into three types. Type I is characterized by involvement of vaginal mucosa and/or floor in the prolapse that can be detected only by vaginal exploration. Type II is characterized by protrusion of anterior floor and lateral walls of the vagina through the vulvar opening giving protruding mass a shape of pear. In type III, whole of vaginal circumference is protruding out giving it an appearance of doughnut visible outside the vulvar opening.
In most of the cases, vaginal hyperplasia regresses spontaneously during the diestrus, but in some cases it reoccurs during the subsequent estrus (almost in 66% to 100%; Johnston et al., 2001). Ovariohysterectomy is recommended as a prophylactic treatment and to avoid recurrence in these cases (Haji et al., 2018). Treatment of the animal depends upon the degree and type of prolapse and on the breedability of the animal. Since vaginal hyperplasia and vaginal prolapse is inherited in nature hence the animal should preferably not be bred.
Initial treatment schedule include hormonal therapy with GnRH analogue (Gonadotropin releasing Hormone) or hСG (Human Chorionic gonadotropin) analogue that causes induction of ovulation and shortening of the estrus (Johnston et al., 2001). Finally there are various surgical techniques for incising edematous protrusion, which can be used depending on the severity of the condition (Pettit, 1986; Soderberg, 1986).
Case History and Observation
A three year old 15 kg Labrador bitch was presented in Guru Angad Dev Veterinary and Animal Sciences University clinics with the history of whelping one month ago and showing a protruded mass from vagina since last two weeks. Feeding and defecation of the animal was normal. However animal shows straining during urination. Physical examination of the animal reveals it to be in good bodily condition. Protruded mass was investigated for the involvement of bladder. Ultrasonography of the mass reveals no involvement of urinary bladder. Appearance of protruded mass was oval in shape about 5.3 cm x 3.7 cm x 2.1 cm with uniform texture. The mass was attached by stalk with the floor of vagina. After exploring the protruded mass it was concluded as prolapse of vaginal floor along with hypertrophy of vaginal mucosa. Animal was given hormonal treatment i.e. Inj. Chorulon (hCG; MSD Animal Health, Pune, India) at the rate of 500 I.U. subcutaneously for three times every alternate day. Supportive therapy includes Syp. Immunol (Immunomodulator and phagocytosis enhancer; Himalaya, Bengaluru, India) 5 ml per os daily for 20 days, Inj. Polybion (Multivitamins; Merck Biopharma, Darmstadt, Germany) 2 ml I/M once daily for 5 days and Antibiotic cover with Amoxyrum forte (Virbac, Mumbai, India) 500 mg I/M once daily for 5 days. The growth was again re-examined after one week. Animal has responded to the treatment, size of the protruded mass has reduced being 4.7cm x 2.9 cm x 2.0 cm (Figure 1) but the response was not much evident.
Surgical removal of the protruded mass was done under general anesthesia. Premedication was done using Atropine sulphate (AtropinSulphate; Paksons Pharma Pvt. Ltd. Delhi, India) @ 0.04 mg/kg bwI/M followed by induction with Xylazine hydrochloride (Xylaxin; Indian Immunologicals Hyderabad, Telangana) @ 0.5 mg/kg bwI/M and subsequently Ketamine hydrochloride (Aneket; Neon laboratories Ltd., Mumbai India) was administered @ 5 mg/
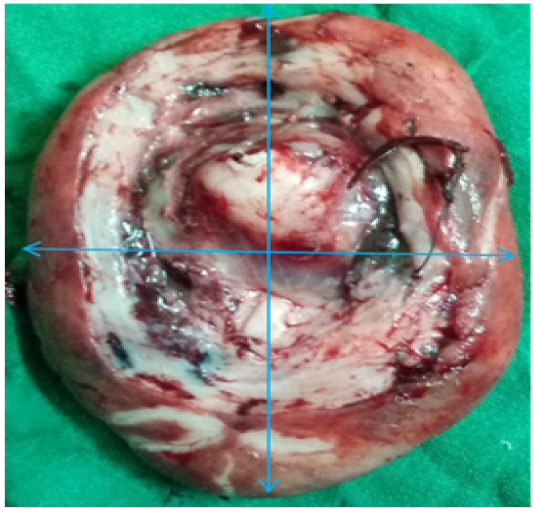
Figure 1: Excised mass

Figure 2: Circumferential bites
-kg bw I/M. Maintenance of animal was done on 0.5-3% Isoflurane (Foran; Ocean Pharamaceuticals; Vadodara, Gujarat: Gaseous Anesthesia). Catheterization of the urinary bladder was done urinary catheter (size 8 gauze number). Clamping of the stalk of protruded mass was done by applying two artery forceps from opposite ends. A 3/8”curved 2.5 cm needle threaded with absorbable Vicryl suture no. 0 (Ethicon) was inserted into the prolapsed mass. Six bites in circumferential clockwise order were taken to secure the blood supply as shown in Figure 2. Suturing was done 2 cm away from the urethral orifice after the artery forceps. Free ends of the corresponding threads were tightened by applying square knot. Protruded mass was carefully excised in a circumferential pattern. Left over stump was sutured by simple interrupted pattern (Figure 3) using absorbable Vicryl suture no. 0 (Ethicon). Vaginal stump was placed in the vagina after topical application of soframycin ointment. The urinary catheter was removed after completion of the surgery. Recovery from anesthesia was speedy and uneventful and further no complications were observed postoperatively. Animal was given cefotaxim (Alkem Laboratories Ltd. Mumbai, India) @ 20 mg/kg bwI/M, meloxicam (Intas Pharmaceuticals Ltd, Ahemdabad, India) @ 4 mg/kg bwI/M and Polybion (Multivitamins; Merck Biopharma, Darmstadt, Germany) 2ml i/m, once daily for five days. Antiseptic dressing of the surgical wound was performed with betadine solution daily for 12 days and the animal recovered successfully.
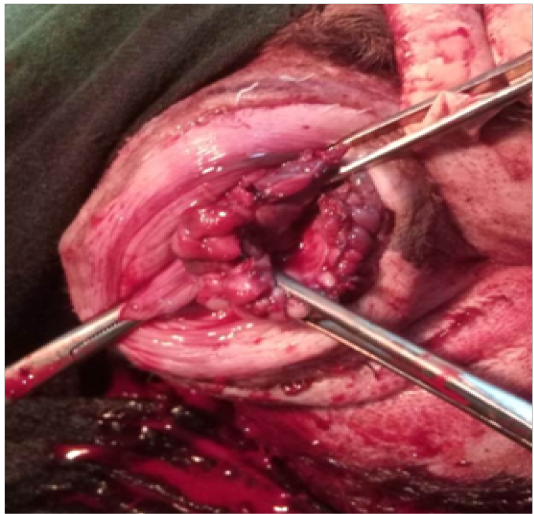
Figure 3: Interrupted suture
Discussion
Occurrence of vaginal prolapse has been described in almost all domestic species. In this case presence of vaginal prolapse along with vaginal hyperplasia is the uncommon part compared to other vaginal issue. Involvement of other organs such as urinary bladder, uterine body or horns, distal colon has also been reported (Wykes, 1986). Ante- partum relaxation of pelvic musculature and increased intra-abdominal pressure can be an etiological factor for pre-partum vaginal prolapse.
Primary objective of every surgical procedure was to combine the experienced performance and best accuracy to minimize bleeding and injury to neighboring organs and further achieved best result. Present case reports modified technique for surgical excision of high grade vaginal prolapse and vaginal hyperplasia in the bitch. This is a modification of the technique described by Schaefers-Okkens (2001). These kinds of cases can be successfully treated with hormonal therapy or surgical excision of the vaginal prolapse and hyperplasia and surgical excision with ovario-hysterectomy (Mostachio et al., 2007). Depending upon the severity of condition and extent of involvement of vaginal folds and mucosa in the present case surgical resection was performed. In the present case, surgical excision was successful and ovariohysterectomy was avoided to maintain the reproductive ability of the animal. Follow up of the case represented healthy recovery without any reoccurrence during subsequent estrus.
Acknowledgements
Authors are thankful to Head, Teaching Veterinary Clinical Complex, Guru Angad dev Veterinary and Animal Sciences University for carrying out the study. No funds were utilized from any grant.
Conflict of interest
Author declares no conflict of interest.
Authors Contribution
Ankit Kumar Ahuja: Operation, manuscript preparation.
Shahbaz Singh Dhindsa: Manuscript preparation and operation.
Ajeet Kumar: Manuscript editing.
Prahlad Singh: Final setting and editing of manuscript.
References
- •





