Journal of Animal Health and Production
Review Article
An Insight into the Pathophysiology, Preventive and Treatment Strategies of Retained Fetal Membranes in Bovines– A Review
Bilal Ahmad Ganaie1*, K. Puhle Japheth2, Murtaza Ali3, Shabir Ahmad Lone1, Shahid Hassan Mir4, Tariq A. Malik4
1Division of Animal Reproduction Gynaecology and Obstetrics, ICAR -National Dairy Research Institute, Karnal-132001, India;2Livestock Production and Management Division, ICAR -National Dairy Research Institute, Karnal-132001; 3Animal Biotechnology Division, ICAR -National Dairy Research Institute, Karnal-132001; 4Animal Nutrition Division, ICAR-National Dairy Research Institute, Karnal-132001, India.
Abstract | Reproductive and productive performance in bovines is adversely affected by the Retention of Fetal Membranes (RFM). For effective management of RFM cases in dairy cattle, understanding of pathophysiology and causes are of critical importance. Hormonally governed processes that are believed to have a role in placental separation are multifactorial and use to come in action before the actual process of birth. This normal process can be interrupted by a diverse set of risk factors, such as abortion, premature or induced parturition, difficult birth (dystocia), hormonal disturbance and immunosuppression, thereby leading to retention of fetal membranes. Current approaches are not in agreement with the success rate attained on practising some classical methods of treatments for RFM. No doubt, systemic administration of antibiotic shave been reported to be successful for treatment of metritis an important consequence of RFM, but such treatment regimens have failed in achieving the desired results in terms of both productivity and reproductivity. Use of immunomodulators, can reduce the chances of uterine inflammation and infection but they are not routinely used. Recently, new treatment approaches, such as administration of collagenase into the umbilical arteries of retained fetal membranes and ozone gas intrauterine have produced desired outcome, but at the same time they are quiet costly too. The current review will try to enlight in detail the progress made in understanding pathophysiology, preventive and treatment strategies of retained fetal membranes in bovines.
Keywords | Retained fetal membranes, Antibiotic, Immunomodulators, Collagenase, Ozone gas
Editor | Asghar Ali Kamboh, Sindh Agriculture University, Tandojam, Pakistan.
Received | April 24, 2018; Accepted | May 16, 2018; Published | June 25, 2018
*Correspondence | Bilal Ahmad Ganaie, Division of Animal Reproduction Gynaecology and Obstetrics, ICAR -National Dairy Research Institute, Karnal-132001, India; Email: bilalganaie32@gmail.com
Citation | Ganaie BA, Japheth KP, Ali M, Lone SA, Mir SH, Malik TA (2018). An insight into the pathophysiology, preventive and treatment strategies of retained fetal membranes in bovines– a review. J. Anim. Health Prod. 6(2): 62-72.
DOI | http://dx.doi.org/10.17582/journal.jahp/2018/6.2.62.72
ISSN (Online) | 2308-2801; ISSN (Print) | 2309-3331
Copyright © 2018 Ganaie et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Retention of fetal membranes has been defined as the failure or delay in separation / expulsion of fetal membranes (Drillich et al., 2006). Fetal membranes or Placenta is a temporary endocrine gland which is virtually expelled at the time of calving. Generally the time gap during which bovines use to expel fetal membranes varied between 8–48 hours following calving (Lee et al.,1989). Moreover, report from one survey revealed that 66 percent of cattle managed to expel the fetal membranes within 6 hours following parturition (Van et al., 1992). Retention of placenta is reported as one of the prevalent complication occurring in animals after parturition. Expulsion of placenta within the stipulated time period is important for subsequent reproductive efficiency as it helps in timely involution and resumption of postpartum cyclicity. Retention leads to a number of problems as it provides a suitable substrate medium for microorganisms to grow on it, causing uterine inflammation, fever, reduced feed intake, weight loss, decreased milk yield, increased days open and subsequently long inter-calving interval. RFM is considered a main risk factor for metritis, so there is likelihood of its occurrence in RFM affected animals.
Incidence
In Friesian cows Esslemont and Kossaibati (1996) has reported an incidence of RFM around 3.6 per cent from the United Kingdom, 24.9 per cent from Egypt by Gaafar et al. (2010) 26 per cent, 16 per cent and 13 per cent in India, respectively in Zebu cows and its crosses, and in Murrah buffalo (Kumari et al., 2015). Increased number of cases occurs during summer months with increased parity, milk yield in the previous seasons and following birth of male fetus (El-Malky et al., 2010). Further Abortions, stillbirths and twin calvings resulted in increased incidence rates of 25.9, 16.4 and 43.8%, respectively (Ahmad et al.,1999).
Sequelae and Economic Importance
Losses incurred due to decreased milk production from RFM result in significant economic drainage to the dairy industry (Dubuc et al., 2011; Kumari et al., 2015). It has been reported that in RFM affected buffaloes milk yield decreased by 239 kg in a single lactation (Kumari et al., 2016). Delayed uterine involution, resumption of ovarian activity, increase in days open, reduction in pregnancy rate McDougall (2001), increase in removal of affected animals Beagley et al. (2010), increased services per conception Holt et al. (1989) and reduction in conception rate and increase in frequency of metritis Gaafar et al. (2010) are the major negative sequelae of RFM. Besides these RFM also cause endometritis, perpural metritis and mastitis in affected animals Bruun et al. (2002) and these diseases in turn compromise fertility and milk yield of dairy animals (Laven and Peters, 1996). In RFM affected cows there is increase in the time of first service by 2-3 days and decrease in conception rate by a factor of 4 – 10, thereby prolonging the calving to conception period by 6-12 days compared to normal ones (Fourichon et al., 2000).
Physiology of Placental Maturation and Separation
The movement and topical bonding of trophoblast cells with uterine epithelial cells result in formation of single cycle with several nuclei (syncytium) that is characteristic of bovine placenta (Peter, 2014). Villi and microvilli interactions at the attachment points (Cotyledon–caruncle junctions) use to facilitate this bond. These interaction points are supplemented with collagen links that have a chief role in loosening of maternal caruncles from fetal attachment points (cotyledons)around the time of calving (Eiler and hopkins,1993).
Role of Hormones
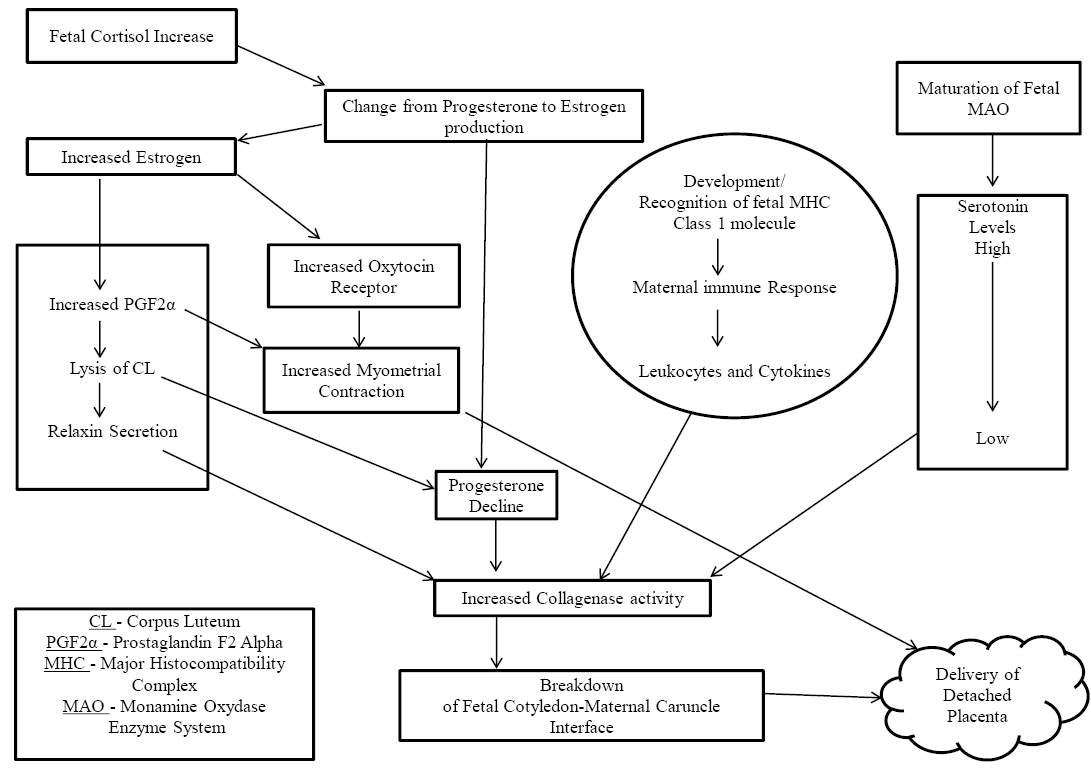
The signals initiating the process of fetal delivery are activated through fetal induction of cortisol which in turn activates conversion of the progesterone into estrogen via. various placental derived enzymes (Flint et al., 1979). Building up of estrogen levels causes increase in oxytocin receptor sensitivity within the myometrium and further lead to increase in PG F2αconcentration (Fuchs et al.,1999). With the drop in progesterone and rise in estrogen levels, prostaglandin synthase 2 (PTGS2) causes synthesis of endometrial PGF2α (Streyl et al., 2012). With PGF2α secretion halt on progesterone production gets released around the parturition time and thereby facilitating myometrial contractions (Shenavai et al., 2012). During advanced stages of pregnancy (nearing calving) the PTGS2 expression has been seen to get initiated from fetal side first, followed by dam side, thus supporting the notion that partrurition starts from the fetal side (Arosh et al., 2004). Increased prostaglandin concentration in turn leads to demise of corpus luteum (CL) (Janszen et al. 1993). CL lysis leads to decline in circulating level of progesterone, as well assecretion of relaxin hormone (Musah et al.,1987). Declining progesterone and increasing relaxin levels in turn used to cause activation of collagenases. Relaxinis responsible for breakdown of collagen fibers, relaxing of the cervix and widening of pelvic ligaments (Engelen et al., 2009). Further, high level of progesterone prevailing during the pregnancy puts ablock on the myometrial contraction and collagenase activity and decline in progesterone near parturition time releases the block necessary for the expulsion of placental membranes (Maj and Kenkofer, 1997). In cattle during gestation the major placental estrogen is Estrone-3-sulfate, Hoffmann and Schuler (2002), with 17-β-estradiol being predominant near the end of gestation. Trophoblastic cells of cotyledons produce estrogen and progesterone, which act upon their corresponding receptors respectively in the caruncular cells (Schuler et al., 2002). This local/confined signalling inturn induces softening of the placentome, cervix, vagina, and related tissues by changing arrangement/orientation of collagen fibres and favouring their water intake (Taverne and Noakes, 2009). Therefore, it is quite clear that the pathways supporting the conventional detachment and dispatch of the placenta areinterrelated/dependent on each other and they use to begin prior to actual time of fetal delivery (Figure 1). Serotonin also plays an important role in regulating bovine placental attachment. During the course of pregnancy high fetal and placental serotonin levels are used to sustain placental attachment through promotion of placental cell proliferation Fecteau and Eiler, (2001), and inhibition of matrix metalloproteinase (MMP) activity (Eiler and Hopkins, 1993). Monoamine oxidase enzyme system maturation close to parturition time metabolizes and subsequently decreases serotonin levels, thereby supporting placental detachment and parturition (Fecteau and Eiler, 2001).
Role of Maternal Immune Responses
Altered hormonal milieu used to support enzymatic decomposition of fetal and maternal attachment points, functioning of the dams immune responses executed towards the fetal membranes play a significant part in the
decomposition process of the placenta. Normal placental expulsion in bovines is characterised by a rise in leukocyte chemotaxis and activity (Kimura et al., 2002). Fetal major histocompatibility complex (MHC-I) molecule identification by dam’s immune system also used to contribute in placental separation and parturition (Davies et al., 2004). During early pregnancy these molecules are missing, but during last trimester of pregnancy fetal trophoblast cells used toexpress them (Davies et al., 2000) and may have an active partin starting of an inflammatory response which used to breakdown the connection points between maternal and fetal parts of the placenta (Davies et al., 2004).Following normal parturition placental retention was recently associated with MHC-I similarity between dam and its developing fetus (Benedictus et al., 2012). From MHC-harmonious pregnancies near term, maternal and fetal macrophages discoloured strongly with antibodies against TNFαvs. macrophages from inharmonious pregnancies (Davies et al., 2004). Broken up DNA, a sign of apoptosis is seen in endometrial epithelial and on the outer cell layer of blastocyst before parturition, in MHC-incompatible caesarean-section samples, but not in compatible pregnancies (Davies et al., 2004). A rise in MHC-I presented patrimonial antigens together with increasing level of estrogeninitiate placentome maturation. Oxytocin synthesis and release, together with increased prostaglandin level support the automatic/ inborn contracting capability of uterine musculature which in turn is important in normal calving (Lye,1996).
Role of Mechanical Forces
Mechanical expulsion of fetal membrane occurs by way of contractions persisting into 3rd stage of labour (Laven and Peters,1996). Active part taken by inbuilt uterine musculature contractions in placental separation/expulsion is meagre till date. However, a pressure change is believed to take place at the fetal side of placentome (cotyledonary villi) by myometrial contractions causing alternate hyperaemic and ischemic situations, and eventually leading to actual separation/release of membranes (McNaughton and Murray, 2009). An abrupt fall of blood flow to the placenta occurs after deliveryof the fetus, resulting in subsequent shrinkage of the villi. The cotyledonary villi collapse by rupture of the umbilical cord during parturition, thereby decreasing the actual area of contact and promoting the dehiscence of fetal membranes. A distortion in the shape of placentomes occurs in such a manner by way of contractile and involuting changes in the uterus, that it promotes the normal and easy passage of fetal membranes (Borel et al., 2006).The present belief is that spontaneous myometrial contraction is important for the final passage of membranes, but main myometrial dysfunction is not necessary for RFM to exist (Grunert, 1986).
Risk Factors and Causes of RFM
Abortion (Muller and Owens,1974), induction of parturition (Terblanche et al. 1976), shortening of gestational length (Joosten et al., 1987), twinning (Muller and Owens,1974), dystocia (Rajala and Grohn,1998), fetotomy (Wehrend et al., 2002), Cesarean section (Joosten et al., 1987), vitamin E, selenium, and carotene defiencies (Julien and Conrad,1976) infectious agents such as Bovine viral diarrhea virus (Niskanen et al.,1995), Brucella, Campylobacter organisms and immunosuppression are the major risk factors for RFM (Laven and Peters, 1996). Mechanism behind biochemical and multi hormonal alterations caused by above mentioned risk factors are not completely understood. These events are believed to be necessary in normal placental separation and delivery, thus suggesting that any sort of intervention with one or more situations can lead to retention of fetal membranes.
Role of Immune Suppression
For successful maintenance of pregnancy suppression of maternal immune responses against the developing fetus is desirable. Significance of immunosuppression in retention of fetal membranes is not fully understood till date (Peter and Bosu, 1987). To avoid rejection of the feto-placental unit during pregnancy, suppression of the immune response is must and any slack in this protective mechanism via disturbance of normal shut down process of immunoprotective mechanisms can subject animal to RFM. Kimura et al. (2002); Pathak et al. (2015) has reported a change in normal functioning of leukocytes (Neutrophils) in terms of their migration/movement towards the site of action on being signalled from the placentomes in those cows which had retention of fetal membranes than those that had not. Immune reactive acid phosphatase content of macrophages is reported to be in high amount in those extracted from placentomes of cows which had already expelled their placentae normally vs. those that have retained placentas (Miyoshi et al., 2002). Further, cows that had RFM vs. those that had normally delivered differ also among cytokine concentrations. A clear association has been reported between occurrence of RFM and 7 mRNA levels of IL-1B, IL-6, IL-8, and TNFα genes at the uterine and placental level Boro et al. (2014), while in RFM cows, pro-inflammatory cytokine (IL1b, IL6, IL8 and TNFα) levels in peripheral blood are reported to be less in comparison to the similar levels 2 to 3 weeks prior to parturition (Kimura et al., 2002; Boby et al., 2013; Boro et al., 2015). IL-10 levels has been reported to differ significantly in RFM affected cows vs.non affected cows (Boby et al., 2013). In contrast, Ras-related protein 7b has been seen to be in low amount in the maternal part of retained placentae, and acts as a negative regulator of Toll-like receptor 9 signalling, prevent the secretion of cytokines supporting inflammation (via. IL-6 and TNF-α) in macrophages, (Kankofer et al., 2015). These finding support the common belief that altered neutrophil and macrophage functioning might beone of the reason responsible for retention of fetal membranes.
Role of Antioxidants
Decreased antioxidant activity of the placenta during pregnancy contributes to RFM (Gupta et al., 2005).Wischral et al. (2001) have reported that dairy cows that had a low placental superoxide dismutase and plasma estrogen prior to parturition developed RFM.A course of actions that is actually believed to be responsible for placental retention is suggested to be linked with disturbed antioxidant capacity at the placenta, decreased estrogen production, which in turn result in decreased PGF2α secretion and slow building up of arachodonic and linoleic acids reserve within placental tissue (Wischral et al., 2001). Vitamin E supplementation in cows used to decrease the incidence of RFM as revealed by multiple (44) scientific researches (Bourne et al., 2007), however the benefits of vitamin E supplementation decider was actual level of vitamin E that was basically present before supplementation of same (Leblanc et al., 2002). Supplementation of vitamin E and selenium has been reported to improve antioxidant potential, increases chemotaxis and leukocyte activity at fetomaternal interface, thereby supporting the normal seperation /expulsion of fetal membranes (Bourne et al., 2007).
Role of Enzymes
Changes in enzyme activity have a significant role in causing RFM, asit is apparent from placentomes of retained vs. non retained placentas that the differences in protease activity exist (Eiler and Fecteau, 2007). Cows that had RFM are having less cotyledon collagenase activity and persistence of type III collagen in them. Besides these, cows that had RFM possess reduced activity of matrix metalloproteinase-9 (MMP-9) and various forms of matrix metalloproteinase-2 (MMP-2) were lacking from them (Maj and Kankofer, 1997). MMP-2 and MMP-9 have a significant part in failure of cotyledon-caruncle attachments and thereby in release/separation of fetal membranes (Eiler and Hopkins,1992). Still now, the exact point/place of secretion of collagenases and other proteases in the cow is mystery, although it is believed that cotyledon or caruncular epithelium and leukocytes might be the possible source (Eiler and Hopkins,1992). Any interruption in normal hormonal changes that used to occur within the uterine environment stop sepithelial cell protease release and further immunosuppression might compromise leukocytic protease activity. So, any one of these conditions can cause RFM by compromising protease activity.
Role of Hormones
Although the exact mechanism is not known, the use of dexamethasone, with or without prostaglandin for induction of labour is considered a common risk factor for development of RFM in cattle (Gross et al.,1985). Collagenase responsible for breakdown of collagen links between fetal and maternal pats of placenta can be prevented by glucocorticoids (Guerin et al., 2004). Further, dexamethasone use to inhibit cotyledonary cell PGF2α synthesis (Izhar et al.,1992).The chances of occurrence of RFM are reduced with the use of PGF2α and dexamethasone, but this combination does not eliminate its total occurrence (Gross et al., 1986). Administration of relaxin along with dexamethasone or cloprostenol during induction of labour, reduces chances of RFM (Musah et al.1987), possibly because relaxin supports collagenase thereby preventing/ slow down the action of dexamethasone.
Role of Calcium
RFM and reduced circulating level of calcium are believed to be associated with one another (Curtis et al., 1983). Melendez et al. (2004) has reported that the dairy cows that were affected with RFM had markedly lower plasma levels of calcium compared to those that had no RFM after they were fed anionic diets. For collagenase activity calcium is required, but in RFM affected cows the blood calcium levels are up to mark from where it can’t prevent the collagenase activity (Gross et al.,1985). Melendez et al. (2003) has reported that supplementation of anionic diets in association with oral calcium and energy proved no longer beneficial in relieving RFM and associated effected. Hypocalcaemia predisposes cows to dystocia Au et al. (1992) and loss of uterine tone, thus interfering with end step of placental separation/loosening, but till now the direct role which calcium used to play in placental separation is not fully known.
Role of Calving Related Problems
Risk factors (dystocia, fetotomy, and caesarean section) for RFM use to impose trauma to the uterus. Edema of chorionic villi uses to occur as a result of trauma that inturn impairs separation of cotyledon-caruncle interface (Laven and Peters,1996). During normal detachment process in bovine placenta, finger-like cotyledonary villi use to get separated at the caruncularpits. But, large /oversized finger like processes (villi) separate from the maternal crypts with difficulty. Heparin is produced from mast cells at the actual area of lesion by trauma induced to the uterus (Gross et al.,1985). Heparin inturn inhibits collagenases (Au et al., 1992), and also delays uterine involution, thereby contribute towards RFM (Eiler and Fecteau, 2007). Difficult birth (Dystocia) and insult to uterine musculature also cause reduction in expulsion/contracting power of same, which in turn can hamper membrane separation /expulsion and lead to secondary retention.
Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDS)
Cows treated with NSAIDS like flunixin meglumine post caesarean section, had higher incidence of RFM compared to non treated cows which is believed to be mediated by way of reduction in prostaglandin synthesis (Waelchli et al.,1999).
Prevention and Treatment Strategies
The objectives behind treatment for retention of fetal membranes is to support early detachment and dehiscence of the membranes so as to reduce the chances of occurrence of metritis, increase chances of early resumption of cyclicity, decrease associated milk losses, reduce reproductive inefficiency, and decrease costs incurred due to veterinarian visits.
Managemental Aspects
Due to multi-factorial nature of non-infectious causes of placental retention and difficulty incurred in diagnosing the same, special care needed to be paid to control measures rather than treatment regimens. The genetic aspect should be given due consideration to select animals with minimal chances for the occurrence of RFM (Ahmed and Zaabal, 2009). To protect from dystocia it is must to select bulls with suitable birth weight for the breed (Skidmore and Loskutoff, 1999).
Nutrition
To have reduced chances of fetal membrane retention supplementation during prepartum period with vitamins and mineral mixture is considered a prophylactic step. Gupta et al. (2005) have reported that 21 days before parturition supplementation with antioxidants, such as 1100 IU of vitamin E (DL -tocopherol acetate) and single intramuscular injection with Se 30 mg (sodium selenite) reduces incidence of RFM in cows.
Manual Removal
Removal of the placenta manually is still commonly practiced method with no beneficial effect on reproduction or milk yield (Drillich et al., 2006). No beneficial effects on reproductive performance were reported when cows were subjected to manual removal and systemic antibiotic treatment when compared without treatment (Drillich et al., 2007). The conclusion drawn out of these treatment strategies is that intrauterine treatment incurs a big loss in terms of cost, extra time veterinary services and antibiotic use. Further, per-vaginal removal of fetal membranes used to have detrimental effects on reproductive efficacy of animal as it increases the chances of secondary infection (Bolinder et al.,1988). Confirmation gained from present literature refrain one from dealing with fetal membranes manually (Peters and Laven,1996). Certainly, with manual removal of placenta, there is more trauma to endometrium, secondly phagocytic activity is reduced (Vandeplassche et al., 1982) and which in turn support bacterial invasion and infection (Peters and Laven,1996). With execution of utmost care still total removal of placenta is very difficult, while with removal of dead /degraded portions bacterial infection can be established (Paisley et al., 1986) or postpartum metritis can setup with ease.
Antiseptics
Chlorhexidine and dilute iodine, have been indicated in the treatment and prevention of RFM. In most cases, their efficacy remains yet to be demonstrated. Special care needs to be taken in using such compounds, especially iodine preparations, as they prove extremely irritating to the endometrium.
Antimicrobials
With the usage of antimicrobials in the treatment of RFM, conflicting results have been reported (Peters and Laven,1996). The aim behind use of antimicrobials in dairy cows affected with RFM is to reduce or relieve animals from metritis andits ill effects on fertility.
Intra Uterine Infusion
Intra uterine infusions with local antimicrobials or usage of boluses have no beneficial effects in reducing the chances of metritis or improving subsequent fertility (Peters and Laven,1996). Cows that were having elevated rectal temperature, when subjected to intrauterine antibiotic treatment have not shown any improvement in postpartum fever. Further cows subjected to different treatment regimens such as no treatment at all (without treatment/ left as such), local administration of antibiotics, manual handling, or a combination of these two had no differences among themselves in terms of both productivity and reproductive efficiency (Drillich et al., 2007). Goshen and Shpigel (2006) has accessed the effect of intrauterine chlortetracycline in the treatment of RFM and clinical metritis in terms of productive and reproductive performance. But desired effect of treatment were seen only in those animal that were having clinical metritis and no difference among treated and untreated animals in terms of both milk output or reproductive performance. These findings point out that intrauterine antibiotics can be fruitful in managing metritis, but there is less likelihood that their use is going to accelerate release/separation of membranes and minimize the chances of metritis in cows having RFM. Intrauterine antibiotics administration has been seen to check uterine bacterial growth, but in doing the same it happens to prevent the necrotizing processes which actually support the detachment/dehiscence of fetal membrane (Roberts, 1986). Intrauterine treatment with tetracyclines in cattle inhibit matrix metalloproteinase (Kaitu’uet al., 2005), and this in turn prevent the normal placental dehiscence/ detachment processes.
Systemic Antimicrobials
Systemic antibiotic administration proves beneficial in RFM cases complicated with fever (LeBlanc, 2008). Systemic antibiotics have been reported to be as successful as systemic antibiotic in association with intrauterine treatment. It has been reported that on systemic treatment with ceftiofur there has been no improvement in membrane shedding/dehiscence, fever recurrence or reproductive performance in animals with or without temperature vs. in those that have had temperature and treated with specific antibiotic accordingly (Drillich et al., 2006).
In acute case of postpartum metritis use of antibiotics has been reported to be helpful (Chenault et al., 2004). Peters and Laven (1996) has reported that the principal cause of decline in fertility in RFM affected cows is metritis. Treatment with 2.2 mg/kg of systemically administered ceftiofur in RFM cases for 5 consecutive days has been seen to be a better option to preclude metritis vs. estradiolor no treatment, although there is no remarkable effect in reproductive performance (Risco and Hernandez, 2003).
Ecbolics
Prostaglandins and oxytocin till date are frequently used hormones in treatment of RFM. RFM cases associated with uterine atony are comfortably treated with these hormonal products as they support the inborn uterine contractions that play a major role in detachment process. Oxytocin is a potent utero kinetic hormone of choice in the immediate postpartum dairy animal. Daily treatment with 20 IU of oxytocin for three to four times have been used for retained fetal membrane (Youngquist and Threlfall, 2007). Stocker and Waelchli (1993) have reported that after caesarean section on treatment with PGF2α about 80% of cows managed to pass out their fetal membranes completely within 12 hours compared to untreated one (58.5%).
Collagenase Treatment
In placental detachment collagen breakdown used to play an important role, so infusion of collagenase proves potent alternative in disintegrating the actual attachment points between fetal and maternal part of placenta (caruncle-cotyledon). Administration of bacterial collagenase @ 200,000 IU dissolved in 1 liter of saline within the umbilical arteries of retained fetal membranes causes prompt detachment of placenta than unpreserved/unprotected ones. It has been seen that 85 per cent (23/27) of dairy cows manage to expel their after births within 36 hours of post calving after treatment with collagenase within 24 to 72 hours. Collagenase treatment strategy is conducted in particular to support/favour cotyledon proteolysis from its corresponding maternal caruncle and it is believed to be more successful than the classical treatments (Eiler and Hopkins,1993). RFM left untreated may undergo autolysis but there are very less/few chances of its expulsion within a week or more post calving (Paisley et al., 1986).
Collagenase therapy has found its application for treatment of retained fetal membranes in a number of species (Haffner,1998). Collagenase treatment results in earlier release of fetal membranes, (Eiler and Hopkins,1993).Till date there has been no evidence of production losses in collagenase treated cases of RFM vs. untreated cows.
Ozone Therapy
Besides the above mentioned traditional approaches, recently new therapies avoiding use of antibiotics are now in practice. Among the newly practiced methods, ozone therapy is one of them. It has been seen that ozone is a strong disinfectant and also possess activity against pathogenic fungi and their spores (Travagli et al., 2009). Ohtsuka et al. (2006) has reported that ozone activates lymphocytes or monocytes, ther by supports secretion of cytokines such as (IFN) α, β, γ, tumour necrosis factor (TNF-α), interleukins (IL) 1, 2, 4, 6, 8 and 10, granulopoietins (GM-CSF) and transforming growth factor β (TGF β) from these cell types. Ozone causes tissue regeneration, favours granulation and epithelialization and also improves the local uterine metabolism.
With ozone therapy there is no bacterial resistance, withdrawal period in milk and meat, which used to be the main the disadvantages with traditional antibiotics (Drillich et al., 2001). In RFM affected cattle the reproductive performance has been evaluated by Djuicer et al. (2012) following the application of two preparations of ozone gas. It has been seen that voluntary waiting period, calving to conception period, overall pregnancy rate and services per conception were improved in treatment group upon application of gas preparations into the uterine body compared to control group.
Immunomodulators
In chronically infertile mares, it has been reported that the E Coli lipopolysaccharide in association with oxytocin produces desired results, as it reduces the occurrence of uterine inflammation and infection, thereby eventually helps in improving their reproductive performance. LPS has been seen to act as a strong signal for endometrial epithelial cells and leukocytes to support the secretion of a number of inflammatory mediators and immuneregulatory cytokines. Nadja et al. (2007) have reported that there is no adverse effect on intrauterine infusion of E coli LPS in mares, thereby supporting its use in expulsion of RFM cases. It has been seen that intra uterine infusion of recombinant human interleukin 8 (rhIL-8) in cattle and mare pulls out/ attracts polymorphonucleocytes into uterus within 6 hours of infusion (Zerbe et al., 2003).
Miscellaneous
The veterinary practices practised by several societies have remarkable effect towards veterinary treatment Lin et al. (2003), because of their cost effectiveness and prominent effects (Mwale et al., 2005). There is a common belief among farmers that the difficult birth and weakness experienced by dairy animal during advanced pregnancy might the reason behind the RFM. It is because of this reason that the mixture of oil and milk is used in such cases as it happens to be a source of energy. However, due to high mineral contents in camel milkit is preferred. The common salt is believed to support the uterine contraction, so it is because of this reason that farmers use to rub it on the back of animal for the smooth expulsion of fetal membranes (Dilshad et al., 2008). Further, Jaggery and turmeric powder mixed with water or Garlic is also given to animal with the common belief that it provides energy and is having cleansing action and thereby helping in fetal membrane expulsion.
Conclusion
In dairy animals RFM represents an important reproductive disorder. RFM compromises the reproductive and productive efficiency of dairy animals causing a great economic loss to the dairy industry. The exact aetiology behind RFM is not fully understood till date. Gaps in understanding of the mechanism involved in placental maturation, cotyledon dehiscence and fetal membranes expulsion are still there. However, improvement in the management and introduction of new remedial and precautionary strategies can help in reducing the incidence of RFM and thereby will help in mitigating the associated losses with RFM.
Conflict of interest
The authors declare that they have no competing interest.
References